Prostate cancer is one of the most prevalent forms of cancer affecting men worldwide. With the increasing incidence of this disease, it has become crucial to develop effective treatment options that can improve patient outcomes and enhance their quality of life. One such promising advancement in prostate cancer therapy is Lutetium-177 PSMA therapy, which has gained significant attention for its remarkable results. In this article, we will delve into the world of Lutetium-177 PSMA therapy, with a particular focus on Pluvicto, an innovative solution in this field.
Prostate cancer is a type of cancer that develops in the prostate gland, a small walnut-shaped organ located below the bladder and in front of the rectum. It is the second most common cancer in men, and its impact on individuals and their families cannot be overstated. Traditional treatment options for prostate cancer include surgery, radiation therapy, and hormonal therapy. However, Lutetium-177 PSMA therapy has emerged as a breakthrough treatment that shows great promise in effectively targeting and treating prostate cancer cells while minimizing damage to healthy tissues.
II. Understanding Prostate Cancer
Before diving into the specifics of Lutetium-177 PSMA therapy, it is important to have a basic understanding of prostate cancer. Prostate cancer occurs when abnormal cells in the prostate gland start to grow and divide uncontrollably. It is often asymptomatic in its early stages, making regular screenings and early detection crucial. Common symptoms of advanced prostate cancer include urinary problems, erectile dysfunction, blood in the urine, and bone pain.
Certain risk factors increase the likelihood of developing prostate cancer, including age (typically affecting men over the age of 50), family history, race (more common in African-American men), and certain genetic mutations. Prostate cancer can be classified into different stages, ranging from localized cancer confined to the prostate to metastatic cancer that has spread to other parts of the body.
III. Lutetium-177 PSMA Therapy: An Overview
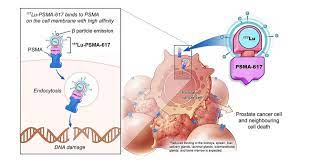
Lutetium-177 PSMA therapy, also known as Lu-177 PSMA therapy or Lutetium PSMA radionuclide therapy, is a targeted therapy designed to treat prostate cancer cells that express the Prostate-Specific Membrane Antigen (PSMA). PSMA is a protein that is highly expressed in prostate cancer cells, allowing for a specific and precise targeting of these cells.
The primary advantage of Lutetium-177 PSMA therapy is its ability to deliver radiation directly to prostate cancer cells, minimizing damage to surrounding healthy tissues. The therapy utilizes a radioactive isotope called Lutetium-177, which emits beta particles that penetrate cancer cells, damaging their DNA and inhibiting their ability to grow and divide.
Pluvicto, an innovative solution in the field of Lutetium-177 PSMA therapy, combines the targeted approach of Lutetium-177 with advanced imaging technology. This allows for accurate visualization and localization of PSMA-expressing cancer cells, enabling precise treatment planning and delivery.
IV. How Lutetium-177 PSMA Therapy Works
Lutetium-177 PSMA therapy involves a series of steps that begin with the identification of prostate cancer cells expressing PSMA. Imaging techniques such as positron emission tomography (PET) scans or single-photon emission computed tomography (SPECT) scans are used to detect and map the distribution of PSMA in the body.
Once PSMA-expressing cancer cells are identified, the patient undergoes a process called radioligand therapy. A ligand molecule, typically a small peptide, is coupled with Lutetium-177 to form a radiopharmaceutical. This radiopharmaceutical is administered to the patient through an intravenous injection.
Pluvicto, an innovative solution in the field of Lutetium-177 PSMA therapy, combines the targeted approach of Lutetium-177 with advanced imaging technology. This allows for accurate visualization and localization of PSMA-expressing cancer cells, enabling precise treatment planning and delivery.
IV. How Lutetium-177 PSMA Therapy Works
Lutetium-177 PSMA therapy involves a series of steps that begin with the identification of prostate cancer cells expressing PSMA. Imaging techniques such as positron emission tomography (PET) scans or single-photon emission computed tomography (SPECT) scans are used to detect and map the distribution of PSMA in the body.
Once PSMA-expressing cancer cells are identified, the patient undergoes a process called radioligand therapy. A ligand molecule, typically a small peptide, is coupled with Lutetium-177 to form a radiopharmaceutical. This radiopharmaceutical is administered to the patient through an intravenous injection.
The Lutetium-177 emits beta particles, which have a short range of penetration, allowing for precise targeting of cancer cells. These particles deliver radiation directly to the PSMA-expressing cancer cells, leading to their destruction. The therapeutic effect of Lutetium-177 PSMA therapy is further enhanced by the cross-fire effect, where radiation emitted from neighboring cells can also damage adjacent cancer cells.
V. Clinical Efficacy of Lutetium-177 PSMA Therapy
Clinical trials and studies have shown promising results regarding the efficacy of Lutetium-177 PSMA therapy in treating prostate cancer. In one study, patients with metastatic castration-resistant prostate cancer who received Lutetium-177 PSMA therapy demonstrated a significant increase in progression-free survival compared to standard treatments. Additionally, the therapy has shown favorable outcomes in terms of overall survival rates and quality of life improvements.
Patient testimonials and experiences further support the positive outcomes of Lutetium-177 PSMA therapy. Many individuals have reported a reduction in pain, stabilization of the disease, and improved quality of life after undergoing Lutetium-177 PSMA therapy. These testimonials highlight the potential of this treatment option to provide meaningful benefits for prostate cancer patients.
VI. Safety and Side Effects
Lutetium-177 PSMA therapy is generally well-tolerated, with manageable side effects. The most common side effects include fatigue, dry mouth, nausea, and decreased blood cell counts. However, these side effects are typically temporary and subside after treatment. Close monitoring and personalized care are essential during Lutetium-177 PSMA therapy to ensure patient safety and manage any potential side effects effectively.
Radiation safety measures are also implemented to protect healthcare providers and minimize radiation exposure. Specialized facilities equipped with shielding and radiation safety protocols are utilized for administering Lutetium-177 PSMA therapy.
VII. The Future of Lutetium-177 PSMA Therapy
The field of Lutetium-177 PSMA therapy is constantly evolving, with ongoing research aimed at further optimizing and improving treatment outcomes. Researchers are exploring combination therapies that combine Lutetium-177 PSMA therapy with other treatment modalities such as immunotherapy or targeted therapies. These synergistic approaches have the potential to enhance the effectiveness of treatment and overcome resistance mechanisms.
The availability of Lutetium-177 PSMA therapy is gradually increasing as more healthcare facilities adopt this advanced treatment option. As research progresses and the therapy becomes more widely accepted, there is hope for improved access to Lutetium-177 PSMA therapy for prostate cancer patients worldwide.
VIII. Conclusion
Lutetium-177 PSMA therapy, including Pluvicto, represents a significant advancement in the treatment of prostate cancer. Its targeted approach and ability to deliver radiation directly to PSMA-expressing cancer cells make it a promising option for patients. Clinical studies have demonstrated its efficacy in prolonging survival and improving quality of life.
As the field of Lutetium-177 PSMA therapy continues to evolve, it holds the potential to revolutionize prostate cancer treatment. It is essential for patients and healthcare professionals to stay informed about this innovative therapy and consider it as a viable treatment option for prostate cancer.